[Rev. 8/6/2022 5:54:52 PM]
[NAC-585 Revised Date: 4-12]
CHAPTER 585 - DRUGS AND COSMETICS
MANUFACTURE OF DRUGS
General Provisions
585.010 Scope.
585.020 Definitions.
585.030 “Active ingredient” defined.
585.040 “Amygdalin,” “laetrile” defined.
585.050 “Batch” defined.
585.060 “Board” defined.
585.070 “Commissioner” defined.
585.080 “Component” defined.
585.090 “Drug” defined.
585.100 “Inactive ingredient” defined.
585.110 “In process” defined.
585.120 “Licensee” defined.
585.130 “Lot” defined.
585.140 “Lot number” defined.
585.150 “Mixup” defined.
585.160 “Procaine hydrochloride with preservatives and stabilizers” defined.
585.170 “Quality control unit” defined.
585.180 “Strength” defined.
Licensing
585.200 Amygdalin: Ingredients.
585.210 Procaine hydrochloride: Ingredients.
585.220 License required to manufacture, prepare or compound drug; qualifications for license.
585.225 License to manufacture drug other than amygdalin or procaine hydrochloride: Prerequisites to issuance.
585.230 Application for license to operate drug manufacturing plant.
585.240 Application for license to manufacture amygdalin or procaine hydrochloride: Action by Commissioner and applicant.
585.245 Application for license to manufacture, prepare or compound amygdalin or procaine hydrochloride: Action by Board.
585.250 Denial, suspension or revocation of license.
585.260 Fees.
Personnel, Facilities, Equipment and Components
585.270 Qualifications of personnel.
585.280 Exclusion of certain employees from direct contact with drugs.
585.290 Buildings used in manufacturing drugs.
585.300 Requirements for manufacturing facilities.
585.310 Approval of plans for construction, remodeling of plants.
585.320 Use of equipment in production and control.
585.330 Maintenance, construction of equipment.
585.340 Identification of containers, lines and other equipment used during production.
585.350 Containers.
585.360 Filters.
585.370 Cleaning and storing of equipment.
585.380 Components: Storage; handling.
585.390 Components: Examination; testing.
585.400 Components: Identification; use.
585.410 Components: Records.
585.420 Components: Retention of sample.
Controls on Production
585.430 Laboratory controls.
585.440 Security.
585.450 Provisions for control of quality.
585.460 Checks and tests of significant steps in process.
585.470 Tests and checks upon drugs.
585.480 Precautions generally.
585.490 Precautions against contamination.
585.500 Precautions respecting penicillin.
585.510 Review of records before release of batch.
585.520 Investigation of unexplained discrepancies in batches.
585.530 Stability of finished drugs.
585.540 Date of expiration of drugs.
585.550 Control of packaging, labeling operations.
585.560 Labeling: Controls; disclosure.
585.570 Returned drugs.
Records
585.580 Master record of drugs.
585.590 Records of batches.
585.600 Records of distribution.
585.610 Complaints.
585.620 Confidentiality of records.
Enforcement
585.630 Inspections.
585.640 Amygdalin, procaine hydrochloride: Verification of substances; appeal.
TAXATION OF AMYGDALIN AND PROCAINE HYDROCHLORIDE
585.650 Applicability.
585.660 Definitions.
585.670 Reporting of gross receipts by manufacturers.
COSMETICS
585.700 Definitions.
585.705 Use, inspection of equipment.
585.710 Qualifications of employees.
585.715 Exclusion of persons with illness or open lesions.
585.720 Report on adverse effects.
585.725 Buildings used to manufacture cosmetics: Condition; size, construction, location; separate rooms.
585.730 Buildings used to manufacture cosmetics: Space.
585.735 Buildings used to manufacture cosmetics: Lighting, facilities; water; sewage disposal.
585.740 Equipment.
585.745 Submission, approval of plan to construct, remodel plant.
585.750 Procedures for production and control.
585.755 Components: Storage, handling; conformance with specifications.
585.760 Components: Examination; testing.
585.765 Containers.
585.770 Laboratory controls.
585.775 Packaging and labeling.
585.780 Master record of production and control.
585.785 Records of batches.
585.790 Records of distribution of lots.
585.795 Records of complaints.
585.800 Qualifications of applicant for license.
585.805 Application for license.
585.810 Denial, suspension, revocation of license.
585.815 Fees; renewal of license.
585.820 Inspections.
585.825 Examination of records.
585.830 Federal regulations adopted by reference.
585.835 Exemptions.
585.840 Severability.
MANUFACTURE OF DRUGS
General Provisions
NAC 585.010 Scope. (NRS 585.210) The criteria prescribed in NAC 585.010 to 585.640, inclusive, apply in determining whether the methods used in, or the facilities or controls used for, the manufacture, processing, packing or holding of a drug conform to, or are operated or administered in conformity with, current good manufacturing practice to ensure that the drug:
1. Meets the requirements of NRS 585.370 to 585.495, inclusive, as to safety; and
2. Has the identity, strength, quality and purity which it is purported or represented to possess.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 2.1, eff. 5-15-78]
NAC 585.020 Definitions. (NRS 585.210) As used in NAC 585.010 to 585.640, inclusive, unless the context otherwise requires, the words and terms defined in NAC 585.030 to 585.180, inclusive, have the meanings ascribed to them in those sections.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.1, eff. 5-15-78]
NAC 585.030 “Active ingredient” defined. (NRS 585.210)
1. “Active ingredient” means any component which is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment or prevention of disease or to affect the structure or any function of the body of a human being or other animals.
2. The term includes components which may undergo chemical change in the manufacture of the drug and be present in the finished drug product in a modified form intended to furnish the specified activity or effect.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.2, eff. 5-15-78]
NAC 585.040 “Amygdalin,” “laetrile” defined. (NRS 585.210) “Amygdalin” or “laetrile” is defined by the formula contained in NAC 585.200.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.3, eff. 5-15-78]
NAC 585.050 “Batch” defined. (NRS 585.210) “Batch” means a specific quantity of a drug which has uniform character and quality within specified limits and is produced according to a single manufacturing order during the same cycle of manufacture.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.4, eff. 5-15-78]
NAC 585.060 “Board” defined. (NRS 585.210) “Board” means the State Board of Health.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.5, eff. 5-15-78]
NAC 585.070 “Commissioner” defined. (NRS 585.210) “Commissioner” means the Commissioner of Food and Drugs.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.6, eff. 5-15-78]
NAC 585.080 “Component” defined. (NRS 585.210) “Component” means any ingredient used in the manufacture of drugs in dosage form, whether or not the ingredient appears in the finished product.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.7, eff. 5-15-78]
NAC 585.090 “Drug” defined. (NRS 585.210)
1. “Drug” has the meaning ascribed to it in NRS 585.080.
2. The term includes amygdalin (laetrile) or procaine hydrochloride with preservatives and stabilizers.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.8, eff. 5-15-78]—(NAC A 9-17-82)
NAC 585.100 “Inactive ingredient” defined. (NRS 585.210) “Inactive ingredient” means any component other than an active ingredient present in a drug.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.9, eff. 5-15-78]
NAC 585.110 “In process” defined. (NRS 585.210) “In process” means in the course of manufacture. Goods in process are distinguished from raw materials or finished products.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.10, eff. 5-15-78]
NAC 585.120 “Licensee” defined. (NRS 585.210) “Licensee” means a natural person, partnership, association, corporation or other form of business organization which is licensed pursuant to NRS 585.245 or 585.495 to manufacture, compound, process or package any drug.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.11, eff. 5-15-78]—(NAC A 9-17-82)
NAC 585.130 “Lot” defined. (NRS 585.210) “Lot” means a batch or any portion of a batch of a drug or, if the drug is produced in a continuous process, any amount of the drug produced in a unit of time or quantity in a manner that ensures its uniformity. A lot is identified by a distinctive number and has uniform character and quality within specified limits.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.12, eff. 5-15-78]
NAC 585.140 “Lot number” defined. (NRS 585.210) “Lot number” means any distinctive combination of letters or numbers, or both, from which the complete history of the manufacture, control, packaging and distribution of a batch or lot of drug can be determined.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.13, eff. 5-15-78]
NAC 585.150 “Mixup” defined. (NRS 585.210) “Mixup” means a miscalculation or misuse of any component in any step of the manufacture, control, packaging, labeling or distribution of a drug.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.14, eff. 5-15-78]
NAC 585.160 “Procaine hydrochloride with preservatives and stabilizers” defined. (NRS 585.210) “Procaine hydrochloride with preservatives and stabilizers” is defined by the formula contained in NAC 585.210.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.15, eff. 5-15-78]
NAC 585.170 “Quality control unit” defined. (NRS 585.210) “Quality control unit” means any organizational element having the authority and responsibility to approve or reject components, materials in process, packaging materials and final products.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.16, eff. 5-15-78]
NAC 585.180 “Strength” defined. (NRS 585.210) “Strength” means:
1. The concentration of each active ingredient of the drug; or
2. The potency of each active ingredient of the drug, which is the therapeutic activity of the ingredient as indicated by appropriate laboratory tests or by adequately developed and controlled clinical data (expressed, for example, in terms of units by reference to a standard).
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 1.17, eff. 5-15-78]
Licensing
NAC 585.200 Amygdalin: Ingredients. (NRS 439.200)
1. The State Board of Health licenses amygdalin (laetrile) in the form of D-mandelonitrile-B-D-glucosido-6-B-D-glucoside:
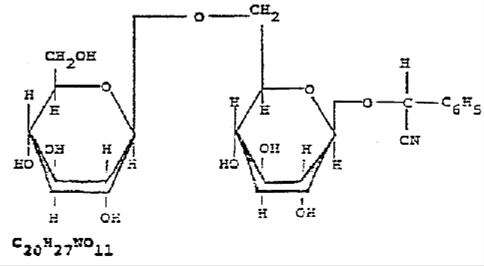
2. No other active ingredient is permitted.
[Bd. of Health, Licensing Reg. §§ 1.1 & 1.1.1, eff. 5-26-78]
NAC 585.210 Procaine hydrochloride: Ingredients. (NRS 439.200)
1. The State Board of Health licenses procaine hydrochloride with preservatives and stabilizers in the form of procaine hydrochloride:
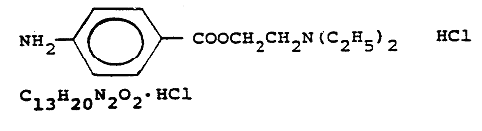
with preservatives and stabilizers.
2. Active ingredients other than procaine hydrochloride are not permitted.
[Bd. of Health, Licensing Reg. §§ 1.2 & 1.2.1, eff. 5-26-78]
NAC 585.220 License required to manufacture, prepare or compound drug; qualifications for license. (NRS 585.210, 585.245)
1. No natural person, partnership, association, corporation or other business organization may manufacture, prepare or compound amygdalin (laetrile) or procaine hydrochloride with preservatives and stabilizers without a license from the State Board of Health.
2. The State Board of Health will not grant a license to manufacture amygdalin or procaine hydrochloride unless the applicant has satisfied the Commissioner that the applicant:
(a) Is a person of good character, honesty and integrity;
(b) Is a person whose background, reputation and associations will not result in adverse publicity for the State of Nevada and its drug manufacturing industry; and
(c) Has adequate business competence and experience for the role or position for which application is made.
3. The State Board of Health will not grant a license to manufacture amygdalin or procaine hydrochloride unless the applicant has satisfied the Commissioner that the proposed financing of the entire operation will be adequate for the nature of the operation and will be obtained from a suitable source. The Commissioner will determine the suitability of the source in accordance with the standards enumerated in subsection 2.
4. No natural person, partnership, association, corporation or other business organization may manufacture, prepare or compound any drug other than amygdalin or procaine hydrochloride without a license from the Commissioner.
5. The Commissioner will not issue a license to manufacture a drug other than amygdalin or procaine hydrochloride unless the applicant has satisfied the Commissioner that the applicant:
(a) Will construct or has constructed a plant in accordance with a set of plans approved by the Commissioner;
(b) Has the competence to manufacture the drug; and
(c) Has the business competence and adequate experience for performing in the role or position for which the application is made.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 7.1-7.2.2, eff. 5-15-78]—(NAC A 9-17-82)
NAC 585.225 License to manufacture drug other than amygdalin or procaine hydrochloride: Prerequisites to issuance. (NRS 585.210, 585.245)
1. An applicant for a license to manufacture a drug other than amygdalin or procaine hydrochloride must submit to the Commissioner for his or her examination and approval:
(a) The formula for the drug, including all its components; and
(b) The procedures to be used in processing the drug.
2. Before the Commissioner will issue such a license, the Commissioner must be satisfied, based on information presented by the applicant, that the applicant has the ability to meet the requirements of this chapter.
(Added to NAC by Comm’r of Food & Drugs, eff. 9-17-82)
NAC 585.230 Application for license to operate drug manufacturing plant. (NRS 585.210, 585.245)
1. An application for a license to operate a drug manufacturing plant must be completed and sent to the Commissioner.
2. The applicant must provide the Commissioner with complete information regarding ownership and must report promptly all significant changes in ownership. If the applicant is a publicly held corporation, only the information regarding the person holding a majority interest need be so provided.
3. A corporate applicant must provide the Commissioner with the name and address of each of its officers, directors and managers. An applicant who is not a corporation must provide the Commissioner with the name and address of each of his or her managerial employees. An applicant shall notify the Commissioner of any change in this information.
4. An applicant must state the proposed hours of operation of the plant. The applicant shall notify the Commissioner of any change in the hours of operation.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 7.3-7.3.4, eff. 5-15-78]
NAC 585.240 Application for license to manufacture amygdalin or procaine hydrochloride: Action by Commissioner and applicant. (NRS 585.210, 585.245, 585.495)
1. The Commissioner will give a notice of hearing by letter to each applicant desiring to manufacture amygdalin (laetrile) or procaine hydrochloride with preservatives and stabilizers, stating the time and place for consideration of the application. The applicant must appear at the hearing. The Commissioner will notify the applicant in writing of the disposition of his or her application.
2. An applicant must submit the formula, including all components, for review and approval to ensure compliance with the formula contained in the license issued by the Board. An applicant must also submit the procedures to be used in processing the drug for review and approval to ensure that the formula contained in the license issued by the Board is not altered thereby.
3. An applicant shall cooperate fully in any background, financial or other investigation made to ensure the accuracy and truthfulness of the information supplied to the Commissioner. The investigation will be conducted before the issuance of any license.
4. An applicant is required to satisfy the Commissioner of its ability to meet the requirements of NAC 585.010 to 585.640, inclusive, before the issuance of any license.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 7.3.5-7.3.8, eff. 5-15-78]
NAC 585.245 Application for license to manufacture, prepare or compound amygdalin or procaine hydrochloride: Action by Board. (NRS 439.200)
1. The Board will consider an application for a license required pursuant to NAC 585.220 to manufacture, prepare or compound amygdalin (laetrile) or procaine hydrochloride with preservatives and stabilizers, and the written recommendation of the Commissioner regarding the application, at a public hearing held:
(a) At the time and place of the next regularly scheduled meeting of the Board;
(b) At the next meeting of the Board that is scheduled in Reno or Las Vegas, whichever city is requested by the applicant; or
(c) As soon as the schedule of the Board permits.
2. The Board is not required to follow the written recommendation of the Commissioner regarding the application.
3. At the public hearing, the applicant and the Commissioner may address the Board and answer any questions of the Board regarding the application.
4. In addition to complying with the requirements set forth in NAC 585.220, the Board will not grant a license to manufacture, prepare or compound amygdalin (laetrile) or procaine hydrochloride with preservatives and stabilizers unless the applicant has satisfied the Commissioner that the applicant has complied with the requirements for the issuance of such a license that are adopted by the Commissioner.
5. At the conclusion of the presentations by the applicant and the Commissioner, the Board will render a decision granting or denying the application. The Board will notify the applicant in writing of its findings of fact, conclusions of law and decision regarding the application as soon as practicable after the date of the hearing.
(Added to NAC by Bd. of Health, eff. 10-30-97)
NAC 585.250 Denial, suspension or revocation of license. (NRS 585.210, 585.245)
1. A failure or refusal of an applicant or a licensee to comply with any provision of NAC 585.010 to 585.640, inclusive, is a ground for denial, suspension or revocation of his or her license.
2. Notice of any denial, suspension or revocation of a license will contain the legal authority and reasons for the action and will be sent to the applicant or licensee by certified mail within 10 days after the action.
3. Within 30 calendar days after receipt of a notice of denial, suspension or revocation, the applicant or licensee may file a notice of appeal with the State Health Officer.
4. Within 30 calendar days after receiving a notice of appeal, the State Health Officer will hold a hearing on the appeal.
5. The State Health Officer will give the applicant or licensee notice of a hearing on appeal at least 15 working days before the date set for the hearing.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 7.4-7.4.4, eff. 5-15-78]
NAC 585.260 Fees. (NRS 585.210, 585.245)
1. An applicant for a license to manufacture amygdalin or procaine hydrochloride must pay an initial licensing fee of $30,000 for the first year to the Commissioner before a license will be issued. To provide for receipt of licensing fees on the basis of fiscal years beginning July 1 and ending June 30, the Commissioner will prorate an initial licensing fee for the period from the date of issuance until the end of the current fiscal year.
2. A license to manufacture amygdalin or procaine hydrochloride is effective until June 30 of the fiscal year in which it is issued, but it is renewable on July 1 of each year. The fee for renewal is $30,000. The Commissioner will prorate a fee for a renewal if it is for part of a fiscal year. An application for a renewal of a license must be received by the Commissioner 30 days before the expiration of the license.
3. An applicant for a license to manufacture a drug other than amygdalin or procaine hydrochloride must pay an initial licensing fee of $2,000 for the first year to the Commissioner before a license will be issued. To provide for receipt of licensing fees on the basis of fiscal years beginning July 1 and ending June 30, the Commissioner will prorate an initial licensing fee for the period from the date of issuance until the end of the current fiscal year.
4. A license to manufacture a drug other than amygdalin or procaine hydrochloride is effective until June 30 of the fiscal year in which it is issued, but it is renewable on July 1 of each year. The fee for renewal is $2,000. The Commissioner will prorate the fee for a renewal if it is for part of a fiscal year. An application for a renewal of a license must be received by the Commissioner 30 days before the expiration of the license.
5. An amount paid as a licensing fee, whether an initial fee or a fee for renewal, is not refundable.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 7.5-7.5.2, eff. 5-15-78; A 12-20-79]—(NAC A 9-17-82)
Personnel, Facilities, Equipment and Components
NAC 585.270 Qualifications of personnel. (NRS 585.210, 585.245, 585.495)
1. The personnel who are responsible for directing the manufacture and control of a drug must be adequate in number and have sufficient education, training and experience, or a combination thereof, to ensure that the drug will have the safety, identity, strength, quality and purity that it is purported or represented to possess.
2. All personnel must have:
(a) Capabilities commensurate with their assigned functions;
(b) A thorough understanding of the manufacturing or control operations which they perform;
(c) The necessary training or experience; and
(d) Adequate information concerning the reasons for the application of the pertinent provisions of this chapter to their respective functions.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 2.3 & 2.3.1, eff. 5-15-78]—(NAC A 9-17-82)
NAC 585.280 Exclusion of certain employees from direct contact with drugs. (NRS 585.210, 585.245, 585.495)
1. A licensee shall exclude from direct contact with drugs or their components any person shown at any time (either by medical examination or supervisory observation) to have an apparent illness or open lesion that may adversely affect the safety or quality of the drugs until the condition is corrected.
2. A licensee shall instruct all his or her employees to report to supervisory personnel any conditions that may have an adverse effect on drugs or their components.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 2.4 & 2.4.1, eff. 5-15-78]
NAC 585.290 Buildings used in manufacturing drugs. (NRS 585.210, 585.245, 585.495)
1. A licensee shall maintain in a clean and orderly condition the buildings used in the manufacture of drugs. The buildings must be of suitable size, construction and location to facilitate adequate cleaning, maintenance and proper operations in the manufacturing, processing, packing, labeling and holding of a drug. Separate rooms may be required to prevent cross-contamination of products.
2. A licensee shall provide buildings with adequate space for:
(a) The orderly placement of equipment and materials to minimize the risk of mixups between different drugs, components of drugs, materials in process, packaging materials, or labeling and to minimize the possibility of contamination;
(b) The receipt, storage and withholding from use of components, pending their sampling, identification and testing before release for manufacturing or packaging;
(c) The holding of rejected components before disposition to preclude the possibility of their use in any manufacturing or packaging procedure for which they are unsuitable;
(d) The storage of components, containers, packaging materials and labeling;
(e) Any manufacturing and processing operations;
(f) Any packaging or labeling operations;
(g) The storage of finished products; and
(h) The control of production and laboratory operations.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 3.1-3.1.1.8, eff. 5-15-78]
NAC 585.300 Requirements for manufacturing facilities. (NRS 585.210, 585.245, 585.495) A licensee shall provide:
1. Adequate lighting (at least 50 foot-candles), ventilation and screening and, if necessary for intended production or control purposes, facilities for adequate control of air pressure, microbiological contamination, dust, humidity and temperature in order to:
(a) Minimize contamination of products by extraneous adulterants, including cross-contamination of one product by dust or particles of ingredients arising from the manufacture, storage or handling of another product;
(b) Minimize dissemination of microorganisms from one area to another; and
(c) Maintain suitable conditions for storage of drug components, materials in process and finished drugs in conformance with information on stability derived pursuant to NAC 585.530.
2. Near working areas adequate locker facilities and hot and cold water washing facilities, including soap or detergent, air drier or single service towels and clean toilet facilities.
3. An adequate supply of potable water under continuous positive pressure in a plumbing system free of defects that could cause or contribute to contamination of any drug. Drains must be of adequate size and, where connected directly to a sewer, must be equipped with traps to prevent back siphonage.
4. Suitable housing and space for the care of all laboratory animals.
5. For the safe and sanitary disposal of sewage, trash and other refuse within and from the buildings and immediate premises.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 3.1.2-3.1.6, eff. 5-15-78]
NAC 585.310 Approval of plans for construction, remodeling of plants. (NRS 585.210, 585.245, 585.495)
1. Before a licensee constructs or extensively remodels a plant for manufacturing drugs, or converts an existing structure for use as such a plant, the licensee must submit the plans to the Commissioner for his or her approval. The plans must include:
(a) The layout and arrangement of the plant;
(b) The materials to be used in construction; and
(c) The location, size and type of fixed equipment and facilities.
2. Approval of plans by the Commissioner does not constitute final approval of the facility. The Commissioner will not issue a license until the Commissioner’s inspection of the facility is made and he or she gives final approval of the facility.
[Comm’r of Food and Drugs, Amygdalin and Procaine Hydrochl. §§ 3.3 & 3.3.1, eff. 5-15-78]—(NAC A 9-17-82)
NAC 585.320 Use of equipment in production and control. (NRS 585.210, 585.245, 585.495) The use of precision, automatic or electronic equipment in the production and control of a drug is permissible if procedures for inspection and checking are used to ensure proper performance.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 2.2, eff. 5-15-78]—(NAC A 9-17-82)
NAC 585.330 Maintenance, construction of equipment. (NRS 585.210, 585.245, 585.495) A licensee shall maintain in a clean and orderly condition all equipment used for the manufacturing, processing, packaging, labeling, holding, testing or controlling of drugs. The equipment must be of suitable design, size, construction and location to facilitate cleaning, maintenance and operation for its intended purpose. The equipment must:
1. Be so constructed that all surfaces which come into contact with a drug or any of its components are not reactive, additive or absorptive in a way which alters the safety, identity, strength, quality or purity of the drug or any of its components.
2. Be so constructed that any substances required for operation of the equipment, such as lubricants or coolants, do not come into contact with any drug in a way which alters the safety, identity, strength, quality or purity of the drug or any of its components.
3. Be constructed and installed to facilitate adjustment, disassembly, cleaning and maintenance to ensure reliability of control procedures, uniformity of production and exclusion from the drugs of contaminants from previous and current operations that might affect the safety, identity, strength, quality or purity of the drug or any of its components.
4. Be of suitable type, size and accuracy for any testing, measuring, mixing, weighing or other processing or storage operation.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 3.2-3.2.4, eff. 5-15-78]
NAC 585.340 Identification of containers, lines and other equipment used during production. (NRS 585.210, 585.245, 585.495) Each container, line and other piece of equipment used during the production of a batch of a drug must be properly identified at all times to indicate accurately and completely its content and, when necessary, the stage of processing of the batch.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 4.1.2, eff. 5-15-78]
NAC 585.350 Containers. (NRS 585.210, 585.245, 585.495)
1. A licensee shall use suitable specifications, test methods and cleaning procedures and, when necessary, sterilization procedures to ensure that containers, closures and other component parts of drug packages are suitable for their intended use.
2. A licensee shall clean containers for parenteral drugs or products or components of drugs with water which has been filtered in accordance with NAC 585.360.
3. Containers of products and their components must not be reactive, additive or absorptive, so as to alter the safety, identity, strength, quality or purity of the drug or its components beyond the official or established limitations.
4. Containers of products and their components must provide adequate protection against external factors that can cause deterioration or contamination of the drug.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.4-4.4.3, eff. 5-15-78]
NAC 585.360 Filters. (NRS 585.210, 585.245, 585.495)
1. Filters used in manufacturing, processing or packaging the components of any drug for parenteral injection in humans must not release fibers into the drug. No filter containing asbestos or releasing any other fiber may be used in manufacturing, processing or packaging such a drug unless the drug or its component cannot be manufactured without the use of such a filter.
2. Filtration must be accomplished by a filter which does not release fiber, except as provided in subsections 3 and 4.
3. If use of a filter which releases fiber is required, an additional filter which does not release fiber and has a maximum pore size of 0.22 microns (0.45 microns if the manufacturing conditions so dictate) must subsequently be used to reduce the content of any asbestos-form particles in the drug or component.
4. Use of a filter containing asbestos followed by use of a filter which does not release fiber is permissible only if the licensee submits proof to the Commissioner that use of only the latter filter will or is likely to compromise the safety or effectiveness of the drug.
5. For the purposes of this section:
(a) A “filter which does not release fiber” is a filter, other than an asbestos filter, which, after any appropriate pretreatment, such as washing or flushing, will not continue to release fibers into the drug or component being filtered.
(b) A “fiber” is any particle whose length is at least three times greater than its width.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.1.10-4.1.13.1, eff. 5-15-78]
NAC 585.370 Cleaning and storing of equipment. (NRS 585.210, 585.245, 585.495) To minimize contamination and prevent mixups, all equipment, utensils and containers must be thoroughly and appropriately cleaned and properly stored and have previous identification removed between batches or at suitable intervals during continuous production.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 4.1.3, eff. 5-15-78]
NAC 585.380 Components: Storage; handling. (NRS 585.210, 585.245, 585.495)
1. A licensee shall, upon receipt, store and handle in a safe, sanitary and orderly condition all components and other materials used in the manufacturing, processing and packaging of drugs and all materials necessary for maintenance of buildings and equipment.
2. A licensee shall take adequate measures to prevent any cross-contamination of drugs and products of drugs.
3. A licensee shall withhold components from use until they have been identified, sampled and tested for conformance with specifications and are approved for release by the quality control unit.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.2-4.2.3, eff. 5-15-78]
NAC 585.390 Components: Examination; testing. (NRS 585.210, 585.245, 585.495)
1. A licensee shall visually examine each container of components before it is used to detect any damage, contamination or indication of breakage of the seal.
2. A licensee shall take an adequate number of samples from a representative number of component containers from each lot and shall subject the samples to a sufficient number of tests to establish their specific identity.
3. A licensee shall appropriately examine representative samples of components liable to contamination with filth, insect infestation or other extraneous contaminants.
4. A licensee shall test representative samples of all components intended for use as active ingredients to determine whether the strength of the ingredients conforms to the appropriate specifications.
5. The licensee shall subject representative samples of components liable to microbiological contamination to microbiological tests before they are used. The components must not contain microorganisms that are objectionable in view of the intended use of the components.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.3-4.3.5.1, eff. 5-15-78]
NAC 585.400 Components: Identification; use. (NRS 585.210, 585.245, 585.495)
1. A licensee shall, as often as necessary, appropriately identify and retest approved components to ensure that they conform to appropriate specifications of identity, strength, quality and purity at the time of use.
2. A licensee shall rotate approved components in such a manner that the oldest stock is used first.
3. A licensee shall identify and hold rejected components to preclude their use in procedures of manufacturing or processing for which they are unsuitable.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.3.6-4.3.6.3, eff. 5-15-78]
NAC 585.410 Components: Records. (NRS 585.210, 585.245, 585.495) A licensee shall maintain appropriate records, including:
1. A record of the identity and quantity of the component, the name of the supplier, the supplier’s lot number and the date of receipt.
2. A record of examinations and tests performed and rejected components and their disposition.
3. An individual inventory and record for each component used in each batch of a drug manufactured or processed.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.3.7-4.3.7.3, eff. 5-15-78]
NAC 585.420 Components: Retention of sample. (NRS 585.210, 585.245, 585.495) A licensee shall retain an appropriately identified, reserve sample of each active ingredient, consisting of at least twice the quantity necessary for all required tests, except those for sterility and determination of the presence of pyrogens, for at least 2 years after distribution of the last drug lot incorporating the component has been completed, or 1 year after the expiration date of the last drug lot, whichever period is longer. The samples must be representative and adequately identified.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.3.8, 4.5.2.1 & 4.5.3, eff. 5-15-78]
Controls on Production
NAC 585.430 Laboratory controls. (NRS 585.210, 585.245, 585.495)
1. The laboratory controls of a licensee must include the establishment of scientifically sound and appropriate specifications, standards and test procedures to ensure that components, drugs in process and finished products conform to appropriate standards of identity, strength, quality and purity.
2. The laboratory controls must also include the establishment of master records containing appropriate specifications for the acceptance of each lot of drug components, product containers and their components used in the production and packaging of drugs and a description of the sampling and testing procedures used.
3. The records must also provide for appropriate retesting of drug components, product containers and their components subject to deterioration.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.5-4.5.2 & 4.5.2.2, eff. 5-15-78]
NAC 585.440 Security. (NRS 585.210, 585.245, 585.495)
1. Every licensee shall provide effective procedures to prevent theft, diversion or adulteration of processed drugs or their components. The procedures must be registered with and approved by the Commissioner.
2. Every person responsible for security must be registered with the Commissioner and is subject to investigation by the Commissioner to determine his or her suitability for the role.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 9.1 & 9.2, eff. 5-15-78]
NAC 585.450 Provisions for control of quality. (NRS 585.210, 585.245, 585.495) The licensee shall provide for:
1. The establishment of master records, when needed, containing specifications and a description of sampling and testing procedures for in-process drug preparation. The samples must be adequately representative and properly identified.
2. The establishment of master records containing a description of sampling procedures and appropriate specifications for finished drugs. The samples must be adequately representative and properly identified.
3. Adequate provisions for checking the identity and strength of drugs for all active ingredients and for ensuring:
(a) Sterility of drugs purported to be sterile and freedom from objectionable microorganisms for those drugs which should be so because of their intended use;
(b) The absence of pyrogens for those drugs purported to be free of pyrogens;
(c) Not more than minimal contamination of ophthalmic ointments by foreign particles and harsh or abrasive substances; and
(d) The pattern of drug release in products designed for a sustained release is tested by laboratory methods to ensure conformance to specifications for the release.
4. Adequate provision for auditing the reliability, accuracy, precision and performance of laboratory test procedures and laboratory instruments used.
5. A properly identified reserve sample of the finished product. The sample must be stored in the same immediate container and closure system in which the drug is marketed. The sample must consist of at least twice the quantity necessary to perform all the required tests except those for sterility and determination of the absence of pyrogens. The sample must be stored under conditions consistent with the labeling of the product. The licensee shall retain the sample for at least 2 years after the distribution of the drug is completed or at least 1 year after the expiration date of the drug, whichever period is longer.
6. A provision for retaining complete records of all laboratory data relating to each batch or lot of drug. A licensee shall retain such records for at least 2 years after the distribution is completed or 1 year after the expiration date of the drug, whichever period is longer.
7. A provision that animals must be maintained and controlled in a manner that ensures their suitability for their intended use. The licensee shall maintain appropriate records identifying the animals and providing a history of their use.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.5.4-4.5.10.1, eff. 5-15-78]
NAC 585.460 Checks and tests of significant steps in process. (NRS 585.210, 585.245, 585.495)
1. Except as provided in subsection 2, each significant step in the process, such as the selection, weighing or measuring of components, the addition of ingredients during the process, the weighing and measuring during various stages of the process and the determination of the finished yield, must be performed by a competent and responsible person and checked by a second competent and responsible person.
2. If the significant steps are controlled by precision, automatic, mechanical or electronic equipment, the proper performance of the equipment must be adequately checked by one or more competent and responsible persons.
3. A written record of the tests and checks at the significant steps in the process must be made by the person performing these tests and by the person charged with checking these steps. An identification of each step must be recorded immediately following its completion.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.1.1 & 4.1.1.1, eff. 5-15-78]
NAC 585.470 Tests and checks upon drugs. (NRS 585.210, 585.245, 585.495)
1. To ensure the uniformity and integrity of his or her products, a licensee must provide adequate controls during production, such as checks upon the weights and disintegration times of tablets, the adequacy of mixing, the homogeneity of suspensions and the clarity of solutions.
2. Sampling of drugs in process must be done at appropriate intervals and with suitable equipment.
3. A licensee shall test representative samples of all drugs in dosage form before their distribution to determine whether they conform to the applicable specifications.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.1.6-4.1.7, eff. 5-15-78]
NAC 585.480 Precautions generally. (NRS 585.210, 585.245, 585.495) The procedure for production and control must include all reasonable precautions to ensure that the drugs produced will have the safety, identity, strength, quality and purity which they are purported or represented to possess.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 4.1, eff. 5-15-78]
NAC 585.490 Precautions against contamination. (NRS 585.210, 585.245, 585.495)
1. A licensee shall take appropriate precautions to minimize microbiological and other contamination in the production of drugs purporting to be sterile or which, because of their intended use, should be free from objectionable microorganisms.
2. A licensee shall establish appropriate procedures to minimize cross-contamination of drugs while they are being manufactured or stored.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.1.4 & 4.1.5, eff. 5-15-78]
NAC 585.500 Precautions respecting penicillin. (NRS 585.210, 585.245, 585.495)
1. If penicillin is being manufactured on the same premises or with the same equipment as is being used to manufacture products subject to NAC 585.010 to 585.640, inclusive, and the products may reasonably be regarded as susceptible to contamination by penicillin, the licensee shall provide for testing the products to determine whether any of them have become contaminated by the penicillin.
2. A licensee shall not market such products if they are intended for use in humans and they are contaminated with an amount of penicillin:
(a) Equivalent to 0.05 unit or more of penicillin G per maximum single dose recommended in the labeling of a drug intended for parenteral administration; or
(b) Equivalent to 0.5 unit or more of penicillin G per maximum single dose recommended in the labeling of a drug intended for oral use.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.5.11 & 4.5.11.1, eff. 5-15-78]
NAC 585.510 Review of records before release of batch. (NRS 585.210, 585.245, 585.495) Each licensee shall establish procedures for the review and approval of all records regarding production and control, including those for packaging and labeling before the release or distribution of any batch.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. § 4.1.8, eff. 5-15-78]
NAC 585.520 Investigation of unexplained discrepancies in batches. (NRS 585.210, 585.245, 585.495)
1. A licensee shall immediately order a thorough investigation of any unexplained discrepancy or failure of a batch to meet any of its specifications, whether or not the batch has already been distributed.
2. The investigation must be undertaken by a competent and responsible person and must extend to other batches of the same drug and other drugs that may have been associated with the specific failure.
3. The licensee shall make a written record of the investigation, which must include the conclusions and following action.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.1.8.1-4.1.8.1.2, eff. 5-15-78]
NAC 585.530 Stability of finished drugs. (NRS 585.210, 585.245, 585.495) The stability of finished drugs must be ensured. The stability must be:
1. Determined by reliable, meaningful and specific methods of testing;
2. Determined on drugs in the same system of container and closure in which they are marketed;
3. Determined on any dry drug that is to be reconstituted at the time of dispensing (as directed on its labeling) as well as on the reconstituted drug.
4. Recorded and maintained in such a manner that the data on stability may be used to establish expiration dates for the drug.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.6-4.6.1.4, eff. 5-15-78]
NAC 585.540 Date of expiration of drugs. (NRS 585.210, 585.245, 585.495)
1. To ensure that drugs liable to deterioration meet appropriate standards of identity, strength, quality and purity at the time of use, the label of all such drugs must show expiration dates which relate to stability tests performed on the drug.
2. Expiration dates appearing on the drug labeling must be justified by readily available data from stability studies described in NAC 585.530.
3. An expiration date must be related to the appropriate storage condition. The condition must be stated on the labeling wherever the expiration date appears.
4. When a drug is marketed in its dry state for use in preparing a liquid product, the label must bear information concerning the expiration date for the reconstituted product as well as the expiration date for the dry product.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.7-4.7.1.3, eff. 5-15-78]
NAC 585.550 Control of packaging, labeling operations. (NRS 585.210, 585.245, 585.495)
1. A licensee shall control his or her packaging and labeling operations adequately to:
(a) Ensure that only drugs which meet the standards and specifications established in the master production and control records are distributed;
(b) Prevent mixups during filling, packaging and labeling operations;
(c) Ensure that correct labels and labeling are employed for drugs; and
(d) Identify each finished product with a lot number that permits determination of the history of the manufacture and control of the batch.
2. A code for the hour, day or shift is appropriate as a lot number for drugs manufactured or processed by equipment used in continuous production.
3. Packaging and labeling operations must be separated by a partition or sufficient distance from operations on other drugs in a manner adequate to avoid mixups and minimize the possibility of cross-contamination.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 5.1-5.1.1.1, eff. 5-15-78]
NAC 585.560 Labeling: Controls; disclosure. (NRS 585.210, 585.245, 585.495)
1. A licensee shall include the following controls in his or her packaging and labeling operations:
(a) An inspection of the facilities before use to ensure that all drugs and materials which were previously used for packaging and labeling have been removed.
(b) The holding of labels and package labeling upon receipt, pending their review and proofing against an approved final copy by a competent and responsible person, to ensure that they are accurate regarding identity and content and are in conformity with the approved copy before they are released to inventory.
(c) The maintenance and storage of each type of label and package labeling, representing different products, strength, dosage forms or quantity of contents in a manner that will prevent mixups and provide proper identification.
(d) A suitable system for ensuring that only current labels and package labeling are retained and that stocks of obsolete labels and package labeling are destroyed.
(e) Restriction of access to labels and package labeling to authorized personnel.
(f) Avoidance of “gang” printing of cut labels, cartons or inserts where the labels, cartons or inserts are:
(1) For different products or different strengths of the same products; or
(2) Of the same size and have identical or similar format or color schemes.
(g) If “gang” printing is employed, added control procedures must be provided for. In devising these added controls, the licensee shall consider the problems related to sheet layout, stacking, cutting and handling during and after printing.
(h) Strict control of the package labeling issued for use with the drug. Such labeling must be carefully checked by a competent and responsible person for identity and conformity to the labeling specified in the production record for the batch. The record must identify the labeling and the quantities issued and used and must reasonably reconcile any discrepancy between quantities of the finished drug and the quantities of the issued labeling. All excess package labeling which bears lot numbers must be destroyed. If any significant, unexplained discrepancy occurs, the licensee shall carry out an investigation pursuant to NAC 585.520.
(i) Adequate examination or laboratory testing of representative samples of finished products after packaging and labeling to safeguard against any errors in the finishing operations and to prevent distribution of any batch until all required tests have been made.
2. Each label on a container of amygdalin or procaine hydrochloride must state that the drug has not been approved as a drug by the United States Food and Drug Administration or by the State of Nevada.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 5.1.2-5.2, eff. 5-15-78]—(NAC A 9-17-82)
NAC 585.570 Returned drugs. (NRS 585.210, 585.245, 585.495)
1. A licensee shall identify any returned drugs as such and hold them for disposition as follows:
(a) If the conditions under which the returned drugs have been held, stored or shipped (before or during their return) or if the condition of the drugs, their containers, cartons or labeling as a result of the storing or shipping cast doubt upon the safety, identity, strength, quality or purity of the drugs, they must be destroyed or subjected to adequate examination or testing to ensure that they meet all appropriate standards or specifications before being returned to stock for warehouse distribution or repacking; or
(b) If the drugs are neither destroyed nor returned to stock, they may be reprocessed if the final product will meet all its standards and specifications and is approved by the Commissioner.
2. A licensee shall maintain records of any returned drugs and shall indicate the quantity returned, the date and their actual disposition.
3. If the reason the drugs are returned implicates associated batches, the licensee shall make an appropriate investigation in accordance with NAC 585.520.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 4.1.9-4.1.9.4, eff. 5-15-78]
Records
NAC 585.580 Master record of drugs. (NRS 585.210, 585.245, 585.495) To ensure uniformity from batch to batch, a master record for each drug and each batch of drug must be prepared, dated and signed or initialed by a competent and responsible person and must be independently checked, reconciled, dated and signed or initialed by a second competent and responsible person. The master record must include:
1. The name of the drug, a description of the dosage form and a specimen or copy of each label and all other labeling associated with the retail or bulk unit, including copies of the labeling signed or initialed and dated by the person responsible for its approval.
2. The name and weight or measure of each active ingredient per dosage unit or per unit of weight or measure of the finished drug and statement of the total weight or measure of any dosage unit.
3. A complete list of ingredients, designated by names or codes sufficiently specific to indicate any special characteristic of quality, and the following information:
(a) An accurate statement of the weight or measure of each ingredient, regardless of whether it appears in the finished product, except that reasonable variations are permitted in the amount of components necessary in the preparation in dosage form if provisions for such variations are included in the master record;
(b) An appropriate statement concerning any calculated excess of an ingredient;
(c) An appropriate statement of theoretical weight or measure at various stages of processing; and
(d) A statement of the theoretical yield.
4. A description of the containers and closures and the packaging and finishing materials.
5. The manufacturing and control instructions, procedures, specifications, special notations and precautions to be followed.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 6.1-6.1.5, eff. 5-15-78]
NAC 585.590 Records of batches. (NRS 585.210, 585.245, 585.495)
1. A licensee shall prepare a record containing complete information concerning the production and control of each batch of a drug which the licensee produces. He or she shall retain the record for at least 2 years after the distribution of the batch is complete or at least 1 year after the expiration date of the batch, whichever period is longer. The record must identify the specific labeling and lot numbers used on the batch and must be readily available during the retention period.
2. The record must include:
(a) An accurate reproduction of the appropriate master formula. The reproduction must be checked, dated and signed or initialed by a competent and responsible person.
(b) An entry for each significant step in the manufacturing, processing, packaging, labeling, testing and controlling of the batch, including:
(1) The date;
(2) The major equipment and lines employed;
(3) The specific identification of each batch of components used;
(4) The weights and measures of components and products used in processing;
(5) In-process and laboratory control results; and
(6) The identification of the person who actively performs and who directly supervises or checks that step in the operation.
3. A batch number that identifies all the production and control documents relating to the history of the batch and all lot numbers associated with the batch.
4. A record of any investigation made pursuant to NAC 585.520.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 6.2-6.3.4, eff. 5-15-78]
NAC 585.600 Records of distribution. (NRS 585.210, 585.245, 585.495)
1. The procedures for warehouse control and distribution of finished goods must include a system by which the distribution of each lot of a drug can be readily determined to facilitate recall of a drug if necessary.
2. Records within the system must contain the name and address of the consignee, the date and quantity shipped and the lot number of the drug.
3. A licensee shall retain the records of distribution for at least 2 years after distribution of the drug has been completed or 1 year after the expiration date of the drug, whichever period is longer.
4. To ensure the quality of the product, a licensee shall include in his or her warehouse control of finished goods a system whereby the oldest approved stock is distributed first whenever possible.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 6.4-6.4.2, eff. 5-15-78]
NAC 585.610 Complaints. (NRS 585.210, 585.245, 585.495)
1. A licensee shall maintain records of all written and oral complaints regarding each product and notify the Commissioner immediately upon the receipt of any complaint.
2. A licensee shall make an investigation of each complaint pursuant to NAC 585.520.
3. A licensee shall maintain the record of each investigation for at least 2 years after distribution of the drug has been completed or 1 year after the expiration date of the drug, whichever period is longer.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 6.5-6.5.1.2, eff. 5-15-78]
NAC 585.620 Confidentiality of records. (NRS 585.210, 585.245, 585.495) All records acquired or compiled by the Commissioner relating to formulas, processing procedures, earnings, revenue and other internal financial matters of any applicant or licensee are confidential and will not be revealed in whole or in part except:
1. For the necessary administration of NAC 585.010 to 585.640, inclusive; or
2. Upon the order of a court of competent jurisdiction.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 10.1-10.1.2, eff. 5-15-78]
Enforcement
NAC 585.630 Inspections. (NRS 585.210, 585.245, 585.495)
1. The Commissioner will make an initial inspection of a licensee’s plant before the license is granted. At least annually the Commissioner will make such additional inspections as he or she deems necessary for enforcement of chapter 585 of NRS and NAC 585.010 to 585.640, inclusive.
2. A licensee shall permit the Commissioner or his or her agent, after proper identification, to enter at any reasonable time any drug manufacturing, processing or packaging plant of the licensee within the State of Nevada to make an inspection to determine compliance with chapter 585 of NRS and NAC 585.010 to 585.640, inclusive. The licensee shall permit the Commissioner or the agent to examine the records of the establishment to obtain pertinent information.
3. After an inspection is made, the Commissioner will prepare a written report of the inspection and give a copy of the report to the licensee. Whenever the licensee is found not to be in compliance with NAC 585.010 to 585.640, inclusive, the Commissioner will take action pursuant to chapter 585 of NRS.
4. The Commissioner or his or her agent will pick up samples of finished drugs or their components from time to time and take the samples for testing at a laboratory approved by the Commissioner. Samples of finished drugs will be picked up and tested at least two times a year.
[Comm’r of Food & Drugs, Amygdalin and Procaine Hydrochl. §§ 8.1-8.3, eff. 5-15-78]—(NAC A 9-17-82)
NAC 585.640 Amygdalin, procaine hydrochloride: Verification of substances; appeal. (NRS 233B.050, 439.200)
1. The Commissioner of Food and Drugs shall analyze any substance or formulation purporting or represented to be amygdalin (laetrile) or procaine hydrochloride with preservatives and stabilizers to determine whether it is the substance licensed by the State Board of Health.
2. Any denial of a substance purporting or represented to be amygdalin (laetrile) or procaine hydrochloride with preservatives and stabilizers may be appealed to the State Board of Health. The appeal must follow the Board’s regulations governing procedures for seeking variances.
[Bd. of Health, Licensing Reg. §§ 2.1 & 2.2, eff. 5-26-78]
TAXATION OF AMYGDALIN AND PROCAINE HYDROCHLORIDE
NAC 585.650 Applicability. (NRS 360.090, 585.497) NAC 585.660 and 585.670 apply to the substances amygdalin (laetrile) and procaine hydrochloride with preservatives and stabilizers (Gerovital H3) which are licensed for manufacture pursuant to NRS 585.495.
[Tax Comm’n, Amygdalin and Procaine Hydrochloride Reg. part No. 1, eff. 2-5-82]
NAC 585.660 Definitions. (NRS 360.090, 585.497) As used in this section and NAC 585.670:
1. “Gross receipts” means the total amount of the sale of each substance, valued in money, whether received in money or otherwise, without a deduction for any of the following:
(a) The cost of the substance sold.
(b) The cost of the materials used, labor or service, or interest paid, or for losses or any other expense.
(c) The cost of marketing the substance.
(d) The cost of transporting the substance before its sale to the purchaser.
2. “Sale” includes any transfer of title or possession, exchange or barter, whether conditional or otherwise, of a substance for a consideration.
3. “Total amount of the sale” includes:
(a) Any services that are part of a sale; and
(b) All receipts, cash, credits and property of any kind.
[Tax Comm’n, Amygdalin and Procaine Hydrochloride Reg. part No. 1, eff. 2-5-82]
NAC 585.670 Reporting of gross receipts by manufacturers. (NRS 360.090, 585.497)
1. A manufacturer of these substances shall report his or her gross receipts based upon the manufacturer’s designated sales price whether or not the revenue from the sales is actually received by the manufacturer in the quarter covered by the report or in a subsequent quarter. The manufacturer’s report must be made on a form prescribed by the Nevada Tax Commission.
2. No allowance for nonpayment of the sales price by any purchaser may be deducted from the manufacturer’s gross receipts.
3. Any sales of the substances to the manufacturer’s subsidiaries must be included in the manufacturer’s gross receipts at the sales price computed and charged to the subsidiaries without deduction for expenses incurred for intercorporate accounting or transactions.
4. Each manufacturer shall maintain accurate and complete records of all sales of the substances for at least 4 years.
[Tax Comm’n, Amygdalin and Procaine Hydrochloride Reg. part No. 1, eff. 2-5-82]
COSMETICS
NAC 585.700 Definitions. (NRS 585.210, 585.245) As used in NAC 585.700 to 585.840, inclusive, unless the context otherwise requires:
1. “Batch” means a specific quantity of a cosmetic which has a uniform character and quality within specified limits and is produced according to a single manufacturing order during a cycle of manufacture.
2. “Control number” means any distinctive combination of letters or numbers, or both, from which the complete history of the manufacturing, control, packaging and distribution of a lot can be determined.
3. “Licensee” means a person who is licensed by the Commissioner to manufacture, process or package cosmetics.
4. “Lot” means a batch or any portion of a batch of a cosmetic or, if the cosmetic is produced in a continuous process, any amount of the cosmetic produced in a unit of time or quantity in a manner that ensures its uniformity.
[Comm’r of Food & Drugs, Cosmetics § 2, eff. 12-16-82]
NAC 585.705 Use, inspection of equipment. (NRS 585.210, 585.245) A licensee may use precision, automatic or electronic equipment to manufacture, process or package cosmetics if the licensee adequately inspects and checks the equipment to ensure its proper performance.
[Comm’r of Food & Drugs, Cosmetics § 3, eff. 12-16-82]
NAC 585.710 Qualifications of employees. (NRS 585.210, 585.245) A licensee shall ensure that:
1. Employees who are responsible for directing the manufacture and control of a cosmetic have sufficient education, training and experience, or a combination thereof, to ensure that the cosmetic will have the safety, identity, quality and purity that it purports or is represented to possess.
2. Employees must have capabilities which are commensurate with their assigned duties, a thorough understanding of the manufacturing or control operations they perform, the appropriate training or experience and adequate information concerning the reasons for statutes and regulations relating to the manufacture of cosmetics.
[Comm’r of Food & Drugs, Cosmetics § 4, eff. 12-16-82]
NAC 585.715 Exclusion of persons with illness or open lesions. (NRS 585.210, 585.245) A licensee shall exclude from direct contact with any cosmetic any person shown at any time, either by medical examination or supervisory observation, to have an apparent illness or open lesion that may adversely affect the safety or quality of the cosmetic until the condition is corrected.
[Comm’r of Food & Drugs, Cosmetics § 5, eff. 12-16-82]
NAC 585.720 Report on adverse effects. (NRS 585.210, 585.245) A licensee shall instruct all of his or her employees to report to their supervisor any conditions that may have an adverse effect on any cosmetic.
[Comm’r of Food & Drugs, Cosmetics § 6, eff. 12-16-82]
NAC 585.725 Buildings used to manufacture cosmetics: Condition; size, construction, location; separate rooms. (NRS 585.210, 585.245)
1. A licensee shall maintain in a clean and orderly condition the buildings used to manufacture cosmetics. The buildings must be of suitable size, construction and location to facilitate adequate cleaning, maintenance and proper operations in the manufacturing, processing, packing, labeling and storage of a cosmetic.
2. The licensee shall provide any separate rooms which are necessary to prevent a cosmetic product from being contaminated by another.
[Comm’r of Food & Drugs, Cosmetics § 7, eff. 12-16-82]
NAC 585.730 Buildings used to manufacture cosmetics: Space. (NRS 585.210, 585.245) A licensee shall use buildings to manufacture cosmetics which have adequate space for:
1. The orderly placement of equipment and materials to minimize the risk of contamination and confusion between different components, materials in process, packaging materials or labeling materials.
2. The storage of components, containers, packaging materials and labeling materials.
3. Any manufacturing and processing operation.
4. Any packaging or labeling operation.
5. The storage of finished products.
6. The control of production and laboratory operations.
[Comm’r of Food & Drugs, Cosmetics § 8, eff. 12-16-82]
NAC 585.735 Buildings used to manufacture cosmetics: Lighting, facilities; water; sewage disposal. (NRS 585.210, 585.245) A licensee shall provide in a building used to manufacture cosmetics:
1. Adequate lighting of at least 50 foot-candles, and ventilation and screening, if necessary, to:
(a) Minimize the contamination of products by extraneous adulterants, including the cross-contamination of one product by dust or particles or ingredients arising from the manufacture, storage or handling of another product;
(b) Minimize the dissemination of micro-organisms from one area to another; and
(c) Maintain suitable conditions for the storage of components, materials in process and finished cosmetics.
2. Adequate lockers and facilities near working areas with soap and hot and cold water for washing hands.
3. An adequate supply of potable water under continuous positive pressure in a plumbing system which is free of defects that could cause or contribute to the contamination of any cosmetic. Drains must be of adequate size and, where connected directly to a sewer, must be equipped with traps to prevent back siphonage.
4. For the safe and sanitary disposal of sewage, trash and other refuse within and from the building and immediate premises.
[Comm’r of Food & Drugs, Cosmetics § 9, eff. 12-16-82]
NAC 585.740 Equipment. (NRS 585.210, 585.245)
1. A licensee shall maintain in a clean and orderly condition all equipment used in the manufacturing, processing, packaging, labeling, holding, testing or controlling of cosmetics. The equipment must be of a suitable design, size, construction and location to facilitate cleaning, maintenance and operation for its intended purpose.
2. Any equipment must:
(a) Be constructed so that all surfaces which come into contact with a cosmetic are not reactive, additive or absorptive in a way which alters the safety, quality or purity of the cosmetic;
(b) Be constructed and installed to facilitate adjustment, disassembly, cleaning and maintenance and to ensure uniformity of production and the exclusion from the cosmetics of contaminants from previous and current operations that might affect the safety and quality of the cosmetics; and
(c) Be of a suitable type, size and accuracy for any testing, measuring, mixing, weighing or other processing or storage.
[Comm’r of Food & Drugs, Cosmetics § 10, eff. 12-16-82]
NAC 585.745 Submission, approval of plan to construct, remodel plant. (NRS 585.210, 585.245)
1. If, after December 16, 1982, a plant for manufacturing cosmetics is constructed or extensively remodeled, or an existing structure is converted for such a use, an applicant must submit plans to the Commissioner for his or her approval before the work is begun.
2. The plans must include:
(a) The layout and arrangement of the plant;
(b) The materials to be used in construction; and
(c) The location, size and type of any fixed equipment and facilities.
3. The Commissioner’s approval of a plan does not constitute his or her final approval of the facility. An actual inspection of the completed facility must be made before a final approval will be given to the applicant. Until such inspection is made and final approval of the facility is granted, the Commissioner will not issue a license.
[Comm’r of Food & Drugs, Cosmetics § 11, eff. 12-16-82]
NAC 585.750 Procedures for production and control. (NRS 585.210, 585.245)
1. The procedures for the production and control of cosmetics which are adopted by a licensee must include all reasonable precautions, including those contained in this section, to ensure that the cosmetics which are produced will have the safety and quality which they purport or are represented to possess.
2. Each significant step in the manufacturing process, such as the selection, weighing or measuring of components, the addition of ingredients during the process and the determination of the finished yield, must be performed by a competent and responsible person. If these steps are controlled by precision, automatic, mechanical or electronic equipment, the proper performance of the equipment must be adequately checked by at least one competent and responsible person.
3. Each container, line and piece of equipment used during the production of a batch must be properly identified at all times to show accurately and completely its contents and, when necessary, the stage of the processing of the batch.
4. To minimize contamination and prevent confusion, all equipment, utensils and containers must be thoroughly and appropriately cleaned and properly stored. The identification marking for the previous batch must be removed between batches or at suitable intervals during a continuous production.
5. A licensee shall establish appropriate procedures:
(a) To minimize the cross-contamination of cosmetics while they are being manufactured or stored.
(b) For the review and approval of all records concerning production control, including those for packaging and labeling, before the release or distribution of any batch.
[Comm’r of Food & Drugs, Cosmetics § 12, eff. 12-16-82]
NAC 585.755 Components: Storage, handling; conformance with specifications. (NRS 585.210, 585.245)
1. A licensee shall store and handle in a safe, sanitary and orderly manner, all components and other materials used in the manufacturing, processing and packaging of cosmetics and all materials necessary for the maintenance of the buildings and equipment.
2. A licensee may not use any component until it has been identified and examined for conformance with the specifications of the product.
[Comm’r of Food & Drugs, Cosmetics § 13, eff. 12-16-82]
NAC 585.760 Components: Examination; testing. (NRS 585.210, 585.245) A licensee shall:
1. Visually examine each container of components before the components are used to detect any damage or contamination.
2. Appropriately examine samples of components which are susceptible to contamination by filth, insects or other extraneous contaminants.
3. Subject the final product to microbiological tests. The product shall not contain microorganisms that are objectionable.
4. Handle and store approved components in such a manner as to guard against their contamination by other cosmetics or components.
[Comm’r of Food & Drugs, Cosmetics § 14, eff. 12-16-82]
NAC 585.765 Containers. (NRS 585.210, 585.245) The containers which are used by the licensee for his or her products and their components must not be reactive, additive or absorptive so as to alter the safety or quality of the cosmetic. The containers must provide adequate protection against any element which can cause the deterioration or contamination of the cosmetic.
[Comm’r of Food & Drugs, Cosmetics § 15, eff. 12-16-82]
NAC 585.770 Laboratory controls. (NRS 585.210, 585.245) A licensee shall include in his or her laboratory controls, provisions for:
1. The establishment of scientifically sound and appropriate specifications and testing procedures to ensure the quality of the finished cosmetic.
2. Verifying the reliability and accuracy of the laboratory testing procedures and instruments which are used.
3. The retention of a properly identified sample of each finished cosmetic. The sample must be stored in a container which is identical to the container in which the cosmetic is marketed. The sample must contain at least twice the quantity necessary to perform all tests performed, except for net weight content, to ensure quality. The licensee shall retain the sample for at least 2 years.
4. The retention of complete records of all laboratory data relating to each lot for at least 1 year after the distribution of the lot is completed.
[Comm’r of Food & Drugs, Cosmetics § 16, eff. 12-16-82]
NAC 585.775 Packaging and labeling. (NRS 585.210, 585.245)
1. A licensee shall control his or her packaging and labeling operations to:
(a) Ensure that only cosmetics which meet the standards and specifications established in the master record of production and control are distributed;
(b) Prevent confusion during filling, packaging and labeling operations;
(c) Ensure that correct labels and labeling materials are used; and
(d) Identify each finished product with a control number that permits the determination of the history of the manufacture and control of the batch.
2. The licensee may use a code for the day or for the shift as a number for cosmetics which are manufactured or processed by equipment which is used in continuous production.
3. The licensee may not use labels for containers and package labeling until they have been reviewed and proofed against an approved final copy by a competent and responsible person. The person shall ensure that they are accurate regarding identity and content and in conformity with the approved copy before they are released for distribution. The licensee shall establish a suitable system for ensuring that only current labels for containers and package labeling are retained and that obsolete labels and package labeling are destroyed.
[Comm’r of Food & Drugs, Cosmetics § 17, eff. 12-16-82]
NAC 585.780 Master record of production and control. (NRS 585.210, 585.245)
1. To ensure the uniformity of batches, a licensee shall prepare a master record of production and control for each cosmetic and each batch.
2. The record must be dated and signed or initialed by a competent and responsible person and must include:
(a) The name and weight or measure of each ingredient.
(b) A complete list of ingredients, designated by names or codes which are sufficiently specific to show any special characteristic of quality, including statements concerning:
(1) Any calculated amount of excess of an ingredient;
(2) The theoretical weight or measure at the various stages of processing; and
(3) The theoretical yield.
(c) A description of the containers, closures and packaging of finished materials.
(d) The instructions regarding manufacturing and control, and the procedures, specifications, and special notations and precautions to be taken.
[Comm’r of Food & Drugs, Cosmetics § 18, eff. 12-16-82]
NAC 585.785 Records of batches. (NRS 585.210, 585.245)
1. A licensee shall prepare a record which is readily available containing complete information concerning the production and control of each batch which he or she produces.
2. The licensee shall retain the record for at least 2 years after the distribution of the batch has been completed.
3. The record must:
(a) Identify the specific labeling and control numbers used on the batch; and
(b) Note the number that identified all of the documents concerning the production, control and history of the batch and all other control numbers associated with the batch.
[Comm’r of Food & Drugs, Cosmetics § 19, eff. 12-16-82]
NAC 585.790 Records of distribution of lots. (NRS 585.210, 585.245)
1. A licensee shall include in the procedures for the control and distribution of finished goods, a record system by which the distribution of each lot can be readily determined to facilitate the recall of a cosmetic, if necessary. The records must contain the name and address of the consignee, the date of each shipment, the quantity shipped and the lot or control number of the cosmetic. The licensee shall retain these records for at least 2 years after the distribution of the cosmetic has been completed.
2. The licensee shall establish a system whereby the licensee’s oldest approved stock is distributed before newer stock is distributed, whenever possible.
[Comm’r of Food & Drugs, Cosmetics § 20, eff. 12-16-82]
NAC 585.795 Records of complaints. (NRS 585.210, 585.245) A licensee shall maintain records of all written and oral complaints he or she receives regarding each product and shall notify the Commissioner immediately upon the receipt of any such complaint.
[Comm’r of Food & Drugs, Cosmetics § 21, eff. 12-16-82]
NAC 585.800 Qualifications of applicant for license. (NRS 585.210, 585.245) The Commissioner will not issue a license pursuant to NRS 585.245 unless the applicant has satisfied the Commissioner that the applicant is competent and has adequate business experience to conduct the activity for which the application for a license is made.
[Comm’r of Food & Drugs, Cosmetics § 22, eff. 12-16-82]
NAC 585.805 Application for license. (NRS 585.210, 585.245)
1. Any person who desires to operate a plant for the manufacture of cosmetics must submit to the Commissioner an application for a license.
2. The applicant must provide the Commissioner with complete information regarding the ownership of the plant and report promptly all significant changes in its ownership.
3. A corporate applicant must provide the Commissioner with the name and address of each of its officers and managers. An applicant who is not a corporation must provide the Commissioner with the name and address of each of the applicant’s managerial employees. Each applicant must notify the Commissioner of any change in this information.
4. The applicant must:
(a) State the proposed hours of operation of the plant and notify the Commissioner of any change in the hours of operation.
(b) Submit the formula of the cosmetic he or she intends to manufacture, including all components, to the Commissioner for review and approval.
(c) Satisfy the Commissioner of his or her ability to meet the requirements of NAC 585.700 to 585.740, inclusive, of this regulation before the Commissioner will issue any license.
[Comm’r of Food & Drugs, Cosmetics § 23, eff. 12-16-82]
NAC 585.810 Denial, suspension, revocation of license. (NRS 585.210, 585.245)
1. The failure or refusal of an applicant or a licensee to comply with any applicable statutes or regulations is a ground for the denial, suspension or nonrenewal of a license.
2. The Commissioner will include in the notice of any denial, suspension, revocation or nonrenewal of a license the legal authority and reasons for the action. The Commissioner will send the notice to the applicant or licensee by certified mail within 10 days after the action is taken.
[Comm’r of Food & Drugs, Cosmetics § 24, eff. 12-16-82]
NAC 585.815 Fees; renewal of license. (NRS 585.210, 585.245)
1. An applicant must pay an initial licensing fee of $300 to the Commissioner before a license will be issued. The Commissioner will prorate an initial licensing fee for the period from the date of the issuance of the license until the end of the fiscal year.
2. A license is effective until June 30 of the fiscal year in which it is issued and must be renewed by July 1 of each year. The renewal fee is $300. In certain cases, the Commissioner may prorate a renewal fee for part of a fiscal year. An application for the renewal of a license must be received by the Commissioner at least 30 days before the expiration of the license.
3. The fees collected pursuant to this section will not be refunded.
[Comm’r of Food & Drugs, Cosmetics § 25, eff. 12-16-82]
NAC 585.820 Inspections. (NRS 585.210, 585.245)
1. The Commissioner will make an initial inspection of an applicant’s plant before a license is granted and will make such an inspection at least annually. The Commissioner will make such additional inspections as he or she deems necessary for the enforcement of chapter 585 of NRS and this regulation.
2. The Commissioner will give to the licensee a written report of the findings of any inspection the Commissioner makes at the licensee’s factory, warehouse or plant.
[Comm’r of Food & Drugs, Cosmetics § 26 + part § 27, eff. 12-16-82]
NAC 585.825 Examination of records. (NRS 585.210, 585.245) A licensee shall permit the Commissioner to examine the records of the establishment to obtain information which is pertinent to the licensee’s operation.
[Comm’r of Food & Drugs, Cosmetics part § 27, eff. 12-16-82]
NAC 585.830 Federal regulations adopted by reference. (NRS 585.210, 585.245) Parts 700 to 740, inclusive, of Title 21 of the Code of Federal Regulations, as those regulations existed on April 1, 1982, are hereby adopted by reference. A copy of the volume containing these parts may be obtained by mail from the Superintendent of Documents, U.S. Government Printing Office, P.O. Box 979050, St. Louis, Missouri 63197-9000, or by telephone at (866) 512-1800, for the price of approximately $6.
[Comm’r of Food & Drugs, Cosmetics § 28, eff. 12-16-82]
NAC 585.835 Exemptions. (NRS 585.210, 585.245)
1. A licensee may petition the Commissioner for an exemption from a particular provision of NAC 585.700 to 585.840, inclusive.
2. The petition must contain:
(a) The section for which the licensee requests the exemption; and
(b) Facts sufficient to show the Commissioner that, because of the harmless nature of the cosmetic the licensee manufactures, the section should not apply.
3. A decision by the Commissioner is final and may not be appealed.
[Comm’r of Food & Drugs, Cosmetics § 29, eff. 12-16-82]
NAC 585.840 Severability. (NRS 585.210, 585.245) If any provision of NAC 585.010 to 585.840, inclusive, or any application thereof to any person, thing or circumstance is held invalid, the Commissioner intends that the invalidity not affect the remaining provisions or applications to the extent that they can be given effect.
[Comm’r of Food & Drugs, Cosmetics § 30, eff. 12-16-82]